
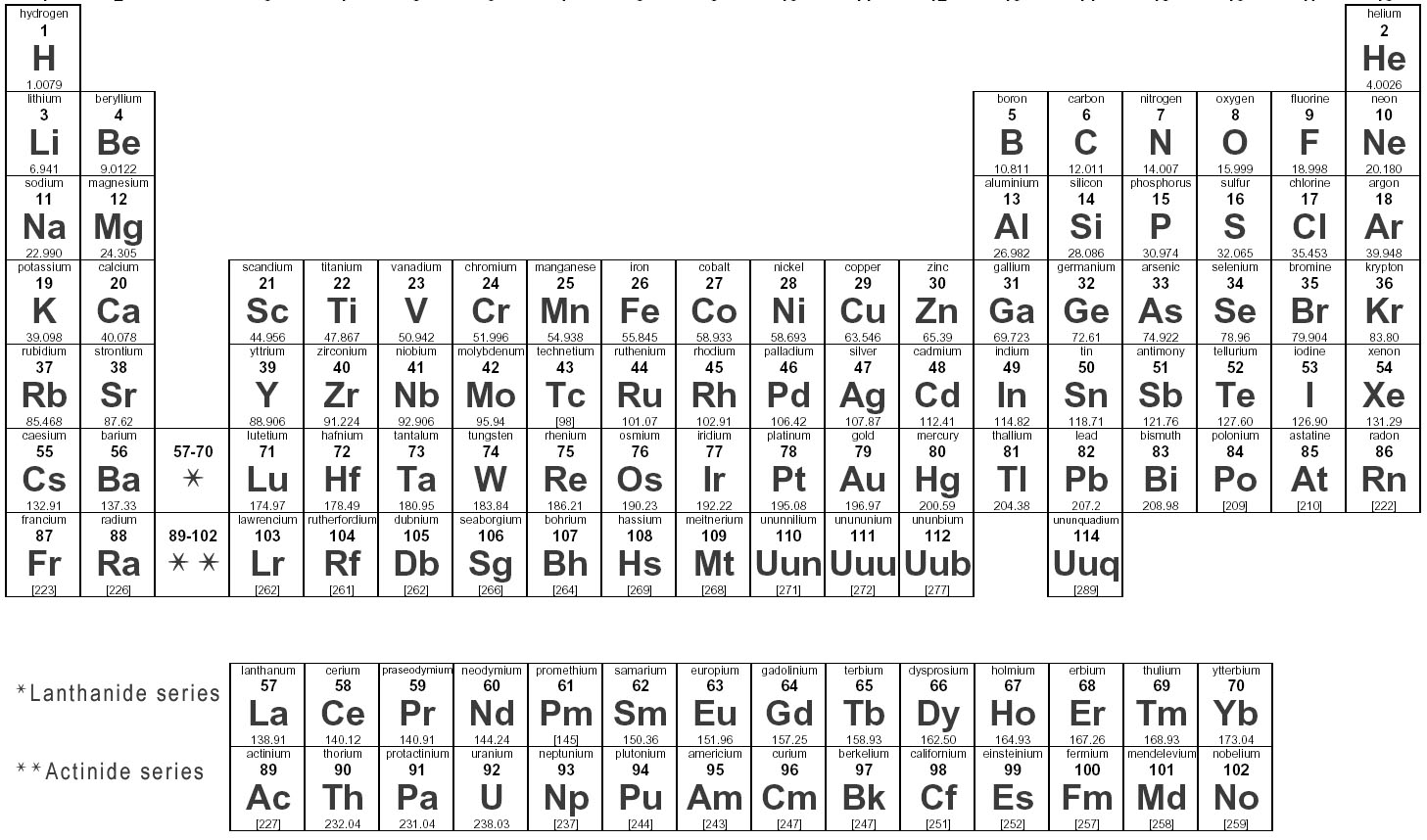
Digital periodic tables, however, are not limited by physical space. Printed periodic tables can only contain a very limited amount of information without becoming extremely large and impractical. Specific details such as atomic weight and electronegativity values can also be found pretty easily. It’s very useful to know the relative properties of the elements and be able to predict their reactivity based on their positions in the periodic table.įor example, you can use the periodic table to predict and compare the ionisation energies of different elements. The periodic table is used by chemists and other scientists as a comprehensive reference source. The metallic characteristics of the elements increase diagonally from right to left. They’re ductile, malleable, and, with the exception of mercury, solid.

The periodic table arranges all known elements into periods and groups that correspond to specific chemical properties.



 0 kommentar(er)
0 kommentar(er)
